MIDDLETON, Wis., July 29, 2025 /PRNewswire/ — Leo Cancer Care, a leader in upright radiotherapy solutions, today announces that its flagship product, Marie®, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA).
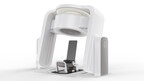
Marie represents a paradigm shift in the delivery of particle therapy, combining an upright patient positioning system and a CT scanner that reimagines the treatment experience for both patients and clinicians, whilst drastically reducing the size and cost compared to existing particle therapy solutions.

“This clearance marks a major milestone not just for Leo Cancer Care, but for the future of cancer treatment,” said Stephen Towe, CEO of Leo Cancer Care. “We’ve long believed there’s a better, more human way to deliver radiotherapy. One that puts the patient at the heart of the treatment experience and embraces smarter, more compassionate design. Marie is the embodiment of that belief.”
Particle therapies such as proton and carbon ion therapy have long been considered the gold standard in radiation oncology. However, the cost, size, and complexity of conventional systems with large rotating gantries have historically limited widespread adoption.
Leo Cancer Care’s upright solution addresses these barriers. By rotating the patient rather than the beam, Marie eliminates the need for a rotating gantry, dramatically reducing infrastructure costs, simplifying installation, and expanding access to this advanced level of care.
Marie is particle beam-agnostic, meaning it’s compatible with a wide range of current and emerging treatment modalities, including proton, carbon ion, BNCT and FLASH therapies. Its upright design opens new possibilities for treatment planning and delivery, particularly for tumors in the thoracic and abdominal regions. When compared to supine treatments, evidence suggests anatomical shifts are reduced due to the gravitational direction.
Through collaborations with leading hospitals around the world, Leo Cancer Care is conducting clinical research and trials, laying a strong foundation for the widespread adoption of upright therapy as a new standard in radiotherapy.
“We are incredibly proud of the teams and collaborators who helped bring Marie to life,” added Thomas ‘Rock’ Mackie, Co-founder and Board Chairman of Leo Cancer Care. “This is just the beginning. We’re working closely with leading particle beam companies and institutions worldwide to explore how upright positioning can unlock new levels of precision and personalization in cancer care.”

Media Contact:
Fiona Redford
Head of Marketing
Leo Cancer Care
[email protected]
![]() View original content:https://www.prnewswire.com/apac/news-releases/leo-cancer-care-receives-fda-510k-clearance-for-marie—a-revolutionary-upright-radiotherapy-platform-302516054.html
View original content:https://www.prnewswire.com/apac/news-releases/leo-cancer-care-receives-fda-510k-clearance-for-marie—a-revolutionary-upright-radiotherapy-platform-302516054.html
SOURCE Leo Cancer Care