The Groundbreaking Accure Laser System is now indicated for the long-term treatment of mild to severe inflammatory acne vulgaris
BOULDER, Colo., Oct. 15, 2024 /PRNewswire/ — Accure Acne, Inc.™ (www.accureacne.com), a pioneer in the development of innovative solutions for the treatment of acne, announced today that it has received a new FDA Clearance K242035 for the long-term treatment of mild to severe inflammatory acne vulgaris.
“This achievement cannot be overstated,” commented Christopher Carlton, Accure Acne, Inc.’s Co-Founder, Chairman of the Board and Chief Executive Officer. “After many years of rigorous technical, scientific, and clinical development, The Accure Laser has delivered yet another milestone achievement-the ability to provide a significant, sustained, and durable drug-free alternative for acne patients and their providers,” continued Mr. Carlton. “This is the vision of Accure Acne and our North Star. Having achieved this, we remain committed to further expanding the use of the Accure Laser and welcome clinicians around the world to partner with us.”
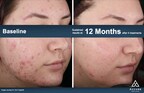
The Accure Laser System unlocks the unique selectivity of the 1726 nm laser wavelength, adding proprietary and first-in-class technology to precisely control thermal gradient depth to have maximum impact on the sebaceous gland. This treat-to-temperature mechanism of action has been validated through multiple IRB-approved clinical trials in the United States, achieving an average inflammatory lesion count reduction of 70% at 6 months after a series of four treatments spaced about one month apart. This was observed for all skin types and severities of acne.
Accure Acne recently completed a limited commercial release earlier this year in the United States, delivering significant clinical outcomes, as well as consistent provider and patient satisfaction scores of over 4.4 out of 5. Since then, Accure has expanded the availability of the Accure Laser System in multiple regions, with first adopters in the Middle East, Europe, The Caribbean, and Asia Pacific, where patients of all severities and skin types can benefit from a safe, effective, and now durable clinical outcome.
“This accomplishment is a testament to the relentless pursuit of this goal by Accure, Quanta System, and the team of clinicians and researchers who have spent many years together to bring the Accure Laser to the world”, added Emil Tanghetti, MD, Founder of The Center for Dermatology and Laser Surgery in Sacramento, CA, and the first Accure Laser investigator in the world. “Our journey in understanding the unique nature of this wavelength and the clinical requirements needed to deliver significant clinical outcomes was certainly challenging. The technical innovations of using temperature as an endpoint, combined with forced air cooling and real-time monitoring algorithms has produced a solution that stands alone from more traditional power-based methods. This unique laser has a platform that can be modified and adjusted to possibly treat a number of other skin conditions. I am excited about the future of this device and technology.”
About Accure Acne, Inc.
At Accure, we are wholly committed to developing revolutionary solutions to eliminate acne. The Accure Laser System was the first 1726 nm-based platform to achieve both CE Mark certification and FDA clearance and is now experiencing rapid adoption in key markets around the world, including the US, Europe, Asia, Caribbean, and the Middle East. We know that acne can have a devastating social, psychological, physical and economic impact on patients around the world, and are pioneering transformative solutions that will continue to address this unmet need and have a positive and profound impact on patients and providers worldwide.
Learn more about Accure and our commitment to acne: www.accureacne.com
Contact:
Vlad Paul-Blanc
(720) 615-3573
[email protected]
View original content:https://www.prnewswire.com/apac/news-releases/accure-acne-announces-new-fda-clearance-for-the-long-term-treatment-of-acne-302276293.html
SOURCE Accure Acne, Inc.