First and only contact force pulsed field ablation (PFA) system engineered to revolutionize care for the hundreds of thousands at risk of death from ventricular tachycardia (VT).FDA’s TAP Pilot and Breakthrough Device Designation are awarded to medical devices that could provide more effective treatment compared to existing approved treatments, reflecting the agency’s commitment to expediting patient access to innovative, safe, and effective therapies.
CARDIFF-BY-THE-SEA, Calif., Dec. 5, 2024 /PRNewswire/ — Field Medical® Inc., a leader in pulsed field cardiac catheter ablation technology, announced today that its FieldForce™ Ablation System has been accepted into the FDA’s Total Product Life Cycle Advisory Program (TAP) Pilot and granted Breakthrough Device Designation for sustained monomorphic scar-related ventricular tachycardia (VT). This recognition underscores the system’s groundbreaking approach to addressing life-threatening VT and the critical unmet needs for patients worldwide.
“The FDA’s TAP Pilot acceptance and Breakthrough Device Designation for the FieldForce™ Ablation System represent pivotal milestones in our journey to regulatory approval,” said Dr. Steven Mickelsen, CEO of Field Medical. “This recognition advances our vision of equipping electrophysiologists with a next-generation, focal PFA tool for fast, accessible VT care.”
Dr. Vivek Reddy, Director of Electrophysiology at Mount Sinai Health System, added, “The FDA’s recognition of this breakthrough technology underscores the urgent need for innovation in treating complex, life-threatening ventricular tachycardia. Field Medical’s ablation system has the potential to redefine VT care for physicians and patients alike.”
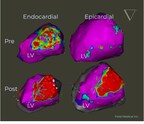
The FieldForce™ Ablation System is the first and only PFA system specifically designed for ventricular arrhythmia ablation. Catheter ablation has been shown to be superior to medical therapy in patients at risk of sudden cardiac death (SCD) from VT (Sapp et al., 2024). SCD, which accounts for approximately 450,000 deaths annually in the United States alone (Mason et al., 2022) is often attributed to VT. Current pharmacological therapies are limited in efficacy, with 30-50% of patients with VT not responding well to traditional drug treatments (Kircher et al., 2023). The emerging science highlights the pressing need for innovative solutions like the FieldForce™ Ablation System.
About Field Medical® Inc.
Founded in 2022, Field Medical is advancing next-generation pulsed field ablation (PFA) technologies to address the complex needs of modern cardiac ablation. The company is led by Dr. Steven Mickelsen, a pioneer in pulsed electric field technology and one of the foremost innovators in the field. Dr. Mickelsen’s foundational work in PFA established the basis for modern advancements in the technology, which Field Medical continues to refine with its groundbreaking solutions. The company’s proprietary FieldBending™ technology and comprehensive product portfolio, including the FieldForce™ Catheter, FieldForce™ Ablation System, and FieldFlex™ Sheath, are designed to expand PFA applications beyond atrial fibrillation to treat complex ventricular arrhythmias. Field Medical is committed to advancing patient outcomes through innovation, transforming cardiac care for the millions impacted by these life-threatening conditions worldwide.
About FieldForce™ Ablation System. The FieldForce Ablation System features a single-point contact force PFA catheter with an innovative design utilizing proprietary FieldBending™ technology to deliver targeted, brief, high-intensity electric fields. This next-generation PFA technology was designed to deliver both precise targeted lesions and large volume transmural lesions in the ventricle.
For more information, visit www.fieldmedicalinc.com and follow us on LinkedIn and X.
The FieldForce™ PFA Ablation System is an investigational device and is limited by Federal (or United States) Law to investigational use.
Media Contact
Holly Windler
619.929.1275
References:
Mason, J., et al. (2022). “Trends in Sudden Cardiac Death Due to Ventricular Tachycardia and Fibrillation in the United States.” Circulation Research.
Kircher, A., et al. (2023). “Efficacy of Antiarrhythmic Medications in the Treatment of Ventricular Tachycardia: A Review.” Journal of the American College of Cardiology.
Sapp, J., et al. (2024). “Catheter Ablation or Antiarrhythmic Drugs for Ventricular Tachycardia.” The New England Journal of Medicine. DOI: 10.1056/NEJMoa2409501.
Photo – https://mma.prnewswire.com/media/2574172/Field_Medical_FieldForce_PFA___Full_Thickness_Endocardial_Ablation.jpg
Logo – https://mma.prnewswire.com/media/2574173/Field_Medical_Logo_Block_WhiteOnBlack_Logo.jpg
SOURCE Field Medical, Inc.