|
SUZHOU, China, Dec. 4, 2025 /PRNewswire/ — On December 3, 2025 (local time in Arizona, USA), ImmVira Group (“ImmVira” or the “Company”) announced a poster presentation at the 26th Annual Meeting of the Society of Urologic Oncology (SUO 2025). The presentation featured the latest interim clinical data (as of September 19, 2025) for its lead HSV-1 oncolytic virus product. MVR-T3011, in high-risk BCG-unresponsive non-muscle invasive bladder cancer (NMIBC) patients.
The patients were enrolled and treated with intravesical MVR-T3011 at two dose levels: 2×109 PFU and 1×1010 PFU in this study. There were a total of 26 patients with papillary (16 patients at the dosage level of 2×109 PFU, 10 patients at the dosage level of 1×1010 PFU), and a total of 12 patients with carcinoma in situ (CIS) (7 patients at the dosage level of 2×109 PFU, 5 patients at the dosage level of 1×1010 PFU) enrolled in this trial. Data from the patients demonstrated a promising efficacy profile:
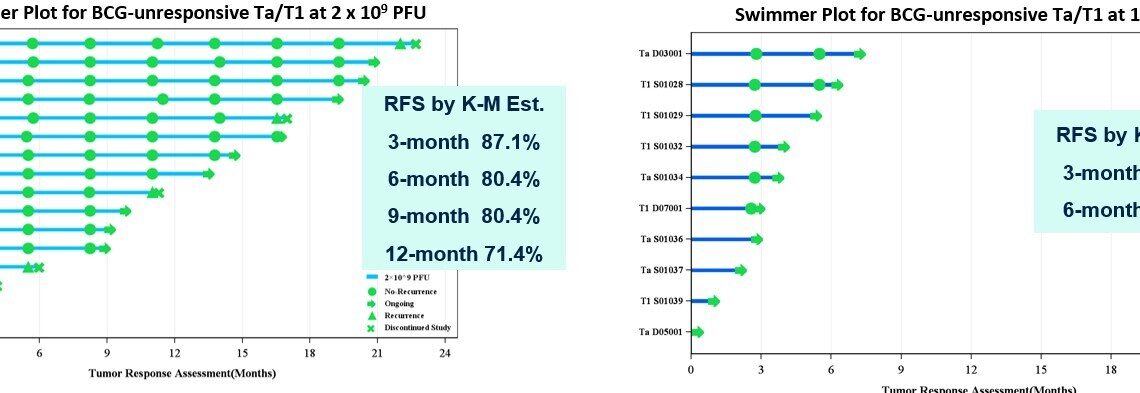
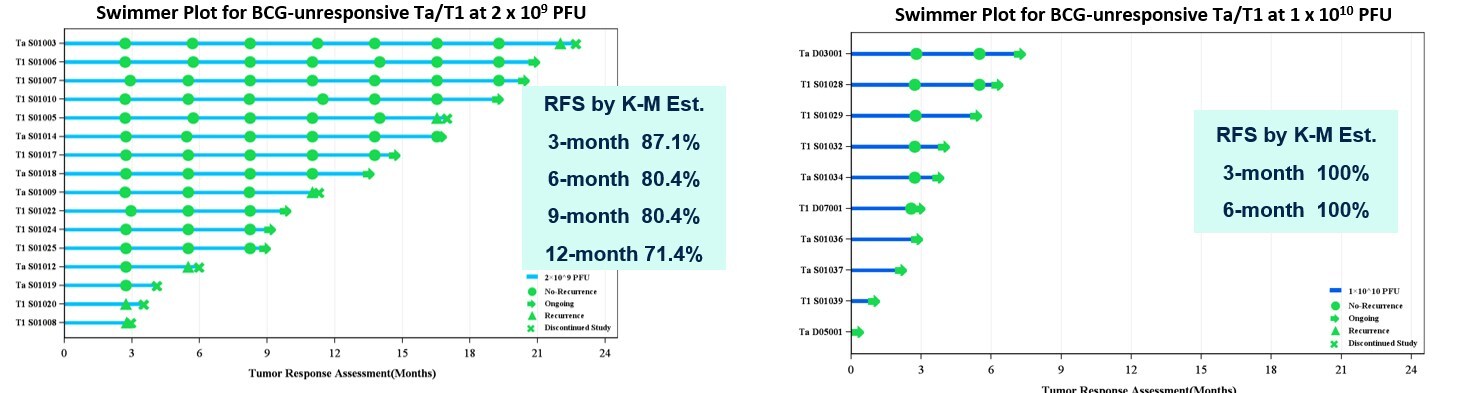
- Among 16 evaluable patients with BCG-unresponsive papillary who received MVR-T3011 at a dose of 2×109 PFU, the 3-month, 6-month, 9-month and 12-month recurrence-free survival (RFS) rates were 87.1%, 80.4%, 80.4% and 71.4%, respectively. Among 6 evaluable patients who received MVR-T3011 at a dose of 1×1010 PFU, 100% of patients remained recurrence free at Month 3 and Month 6.
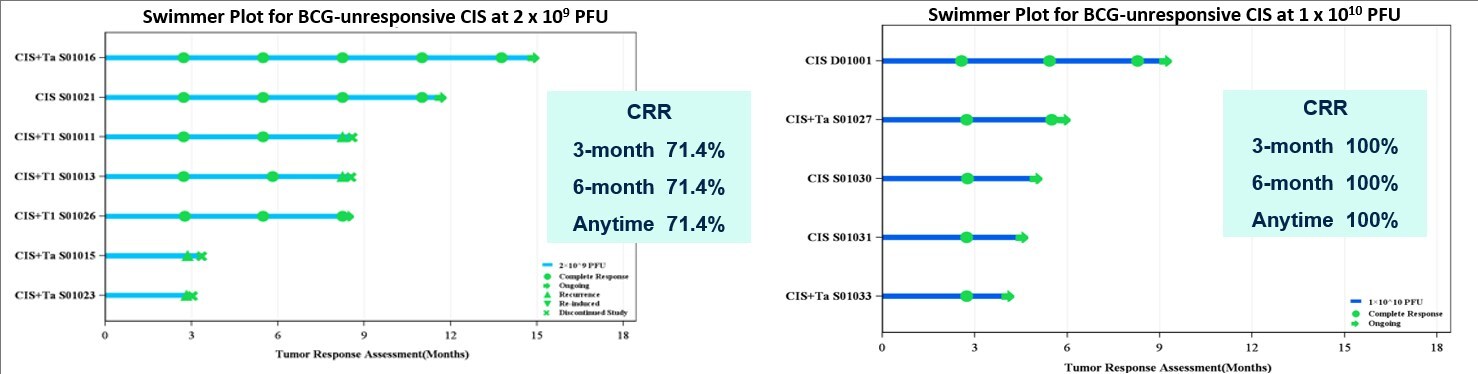
- As of the same date, among 7 evaluable patients with BCG-unresponsive CIS (with or without Ta/T1) who received MVR-T3011 at doses of 2×109 PFU, the CR at any time, 3-month and 6-month CRR was 71.4%, and among 5 evaluable patients with BCG-unresponsive CIS (with or without Ta/T1) who received MVR-T3011 at doses of 1×1010 PFU, the CR at any time, 3-month and 6-month CRR was 100%.
Consistent with previous clinical findings, MVR-T3011 continued to demonstrate a favorable safety and tolerability profile in the latest study. Most treatment-emergent adverse events (TEAEs) were at Grades 1 or 2. Only five Grade 3 TEAEs were reported, two of which were treatment-related adverse events (TRAEs) and were consistent with reactions commonly associated with catheterization procedures. No Grade 3 or above TEAEs and no dose-limiting toxicities (DLT) occurred.
According to Frost & Sullivan, bladder cancer is one of the top 10 most common solid tumors globally by incidence and often requires prolonged treatment and surveillance spanning 5-10 years. NMIBC is a main type of bladder cancer, representing approximately 75% of all newly diagnosed bladder cancer cases. The current standard of care for high-risk NMIBC is Bacillus Calmette-Guerin (BCG). However, the availability of BCG is significantly limited by global supply shortages. In the U.S., BCG supply meets less than 30% of the total demand. These significant unmet medical needs highlight a clear opportunity for novel immunotherapies such as oncolytic viruses, which hold considerable potential as a new mechanism of action in this underserved market.
“We are highly encouraged by the interim efficacy data from the study, especially the high CR and RFS rate for both BCG-unresponsive CIS and papillary patients at 1×1010 PFU,” said Dr. Grace Zhou, Chairwoman and CEO of ImmVira. “We have initiated a phase II trial for BCG-unresponsive high-risk NMIBC in the U.S. in June 2025 and are progressing a global multi-regional clinical trial (MRCT) inclusive of China. We believe MVR-T3011 could emerge as the new generation of therapy for patients with high-risk, BCG-unresponsive NMIBC.”
About MVR-T3011
MVR-T3011, represents a breakthrough in HSV-1-based oncolytic immunotherapy. Its proprietary “3-in-1” design unites a replication-competent, tumor-lytic HSV-1 backbone with anti-PD-(L)1 antibody and IL-12, enabling it simultaneously to lyse tumor cells and stimulate innate and adaptive immunity. MVR-T3011 has demonstrated its adaptability and feasibility across multiple routes of administration including intratumoral, intracavitary and intravenous administrations. MVR-T3011 is the world’s first HSV-1-based oncolytic immunotherapy that has completed a phase I trial via systemic intravenous dosing under the FDA regulatory regime.
About ImmVira
ImmVira is a global leading clinical-stage biotechnology company that is powered by proprietary biological engineering technology, and is dedicated to the discovery, development, manufacture and commercialization of novel oncolytic immunotherapies and engineered exosome therapies. We have strategically designed and built a risk-balanced product portfolio that comprised both potentially best-in-class oncolytic immunotherapy candidates for solid tumors and innovative engineered exosome assets for clinical application or direct commercialization. Driven by our strategic priority to become a global leader in the full spectrum of bladder cancer treatment development, we have adopted a rationalized, adaptive approach to advance oncolytic immunotherapy candidates with high clinical potential globally. In parallel, leveraging our deep expertise in biological engineering, we have pioneered development of engineered exosome candidates targeting chronic, hard-to-treat diseases as well as age-related conditions. These selected engineered exosome assets are being deliberately accelerated through strategic, differentiated regulatory pathways to enable expedited commercialization and generate sustainable cash flows that will fuel our broader drug development efforts.