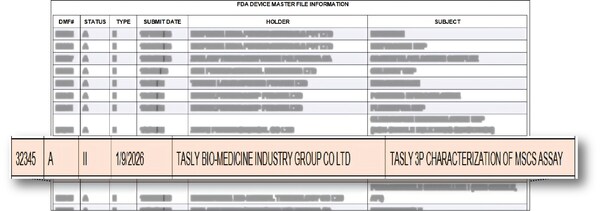
TIANJIN, China, Jan. 26, 2026 /PRNewswire/ — In a landmark move for the cell therapy industry, a Device Master File (DMF) describing the first dedicated quality control standard for Mesenchymal Stromal Cells (MSCs) was accepted by the U.S. Food and Drug Administration (FDA). The agency’s Master File acknowledgement letter, issued on January 9, 2026, incorporates the “Tasly 3P Characterization of MSCs Assay” (MF 32345) into its regulatory framework. This step provides long-sought guidance to ensure the consistent, safe, and effective clinical use of MSCs.
Historically misclassified as stem cells, MSCs have been associated with variable clinical outcomes due to a lack of specific quality benchmarks. The newly recognized ” 3P ” assay directly addresses this by evaluating three core attributes: Property (cell identity), Purity (freedom from contaminants), and Potency (functional activity). This focus ensures that therapeutic MSC products are accurately defined, devoid of heterogeneous cell populations, and biologically potent. The introduction of the Tasly 3P assay marks a pivotal shift toward normalized characterization of MSCs. It mitigates historical risks such as tumor formation and therapeutic inconsistency while empowering clinicians and patients to verify cell quality before treatment. As the first FDA-recognized protocol of its kind, it establishes a new benchmark for the field and accelerates the transition in regenerative medicine from a stem-cell-centric model to a stromal cell-focused paradigm.
By endorsing a standard that aligns with the modern scientific understanding—that MSCs primarily function via paracrine signaling, not differentiation—the FDA is closing a critical regulatory gap. This alignment with updated International Society for Cell & Gene Therapy guidelines is poised to streamline Investigational New Drug applications, enhance clinical trial reliability, and foster global harmonization in MSC product evaluation.