|
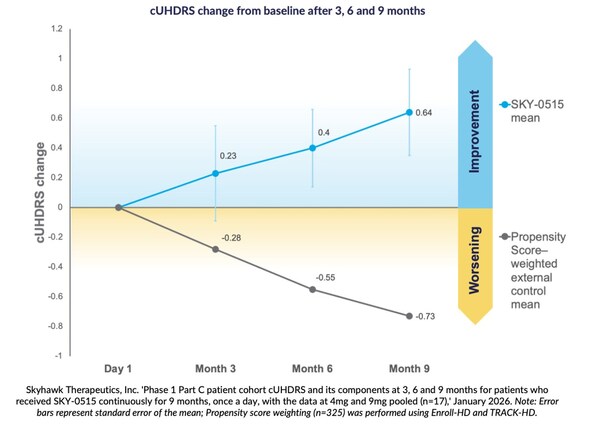
Nine-month findings show mean improvement in Composite Unified Huntington’s Disease Rating Scale from baseline of +0.64 points, compared to natural history expected worsening of cUHDRS in symptomatic patients of -0.73 points over nine months, based on propensity score weighting.
Skyhawk also announces SKY-0515’s Phase 2/3 FALCON-HD trial has expanded worldwide. Skyhawk has now dosed more than 90 patients.
BOSTON, Jan. 27, 2026 /PRNewswire/ — Skyhawk Therapeutics, Inc., a clinical-stage biotechnology company developing novel small molecule therapies to modulate critical RNA targets, today announces positive results from the nine month interim analysis of the Company’s investigational treatment for Huntington’s disease (HD) with SKY-0515.
Treatment with SKY-0515 results in dose-dependent reductions of mHTT protein in blood of 62% at the 9mg dose, and dose-dependent PMS1 mRNA reduction of 26%. PMS1 is a key driver of somatic CAG repeat expansion and HD pathology. SKY-0515 has also demonstrated excellent central nervous system exposure and been generally safe and well tolerated.
At three, six and nine months, patients receiving SKY-0515 in the Part C patient cohort of the Phase 1 clinical trial of SKY-0515, demonstrate mean Composite Unified Huntington’s Disease Rating Scale (cUHDRS) improvement from baseline. At nine months, in a pooled analysis, this improvement is +0.64 points compared to expected worsening at nine months of cUHDRS in symptomatic patients of -0.73 points, based on propensity score weighting using Enroll-HD and TRACK-HD.
“I am very encouraged by these safety and early efficacy data from SKY-0515’s Phase 1 Part C trial in patients, showing divergence in cUHDRS away from expected natural history deterioration at the three, six, and nine month prespecified analyses,” said Ed Wild, Professor of Neurology at University College London. “SKY-0515 continues to reduce mHTT protein to the greatest extent demonstrated by any therapeutic tested to date in patients, with clinical and biomarker data showing the drug is well tolerated at all doses tested. SKY-0515’s ability to reduce both mHTT and PMS1 offers a potent combination for treating Huntington’s disease via two of its core pathogenic mechanisms. These open-label trial results, due to be validated in the ongoing placebo-controlled FALCON-HD trial, give an expectation of meaningful impact for people living with HD across the world – for whom an orally administered huntingtin-lowering treatment such as SKY-0515 will be truly transformative.”
“Our goal for our Phase 1 study was to establish safety and biomarker activity,” said Sergey Paushkin, Head of R&D at Skyhawk Therapeutics, “and the continued strength of SKY-0515’s biomarker response in our nine month interim data analysis – and the improvement in the potential endpoint, cUHDRS, compared to a worsening of the cUHDRS score in the natural history data for patients – underscores SKY-0515’s potential as a best in class disease-modifying therapy for HD. These interim data represent an important milestone for SKY-0515 and highlight the power of Skyhawk’s platform to deliver first-in-class small molecules for devastating diseases with no approved disease-modifying therapies.”
Huntington’s disease is a rare, hereditary, and ultimately fatal neurodegenerative disorder that affects over 40,000 symptomatic patients in the United States, with hundreds of thousands estimated to be affected worldwide. There are currently no approved treatments which slow or halt disease progression. SKY-0515 is an orally-administered, investigational small molecule RNA modulator developed through the company’s novel RNA-modulating platform, SKYSTAR®. SKY-0515 therapeutically reduces both HTT protein and PMS1 protein. PMS1 is an additional key driver of somatic CAG repeat expansion and HD pathology and should complement the benefits of reducing mutant HTT.
Skyhawk also announces today that its SKY-0515 Phase 2/3 FALCON-HD trial, open at twelve sites in Australia and New Zealand, has expanded worldwide. Skyhawk has now dosed more than 90 patients with SKY-0515.
SKY-0515 is the first Skyhawk drug in clinical trials.
Skyhawk expects to put additional small molecule drugs to treat rare neurological diseases with no approved disease modifying therapies in the clinic by the end of 2027.
About SKY-0515’s Phase 1 Clinical Study
SKY-0515’s Phase 1 clinical trial is a first-in-human trial designed to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of SKY-0515 in healthy volunteers and individuals with early-stage Huntington’s disease (HD). The trial is separated into three parts. Parts A and B evaluated SKY-0515 in Healthy Volunteers. Part C is a double-blind placebo-controlled parallel design study of two dose levels of SKY-0515 and placebo in individuals with early-stage HD (HD-ISS Stage 1, 2, or mild Stage 3) for 84 days followed by a 12 month extension of active treatment where all participants will receive either a low or high dose of SKY-0515 in a blinded fashion. The objectives of the study include evaluating mutant HTT protein and PMS1 mRNA. The first patients were dosed in SKY-0515’s Part C in January 2025. Enrollment in Phase 1C of the SKY-0515 trial is now complete.
About SKY-0515’s Phase 2/3 FALCON-HD Clinical Study
FALCON-HD (NCT06873334) is a Phase 2/3 randomized, double-blind, placebo-controlled, dose ranging study to evaluate the pharmacodynamics, safety, and efficacy of SKY-0515 in 120 participants with Stage 2 and early Stage 3 HD across 12 sites in Australia and New Zealand, and 400 participants with Stage 2 and early Stage 3 HD in 40+ worldwide sites. Eligible patients will receive a once-daily oral dose of SKY-0515 at one of three dose levels or placebo, for a treatment period of at least 12 months. The trial aims to assess the potential of SKY-0515 to modulate RNA splicing and reduce mHTT and PMS1 proteins, which are implicated in the pathology of Huntington’s disease. Additional information about FALCON-HD, including participating sites and eligibility criteria, can be found at ClinicalTrials.gov and www.FALCON-HD.com.
About Skyhawk Therapeutics
Skyhawk Therapeutics is a clinical-stage biotechnology company which uses its proprietary platform, SKYSTAR®, to discover and develop small molecule RNA modulating therapies for the world’s most intractable diseases. For more information visit www.skyhawktx.com.
Skyhawk Contact
Maura McCarthy
Head of Corporate Development
[email protected]