|
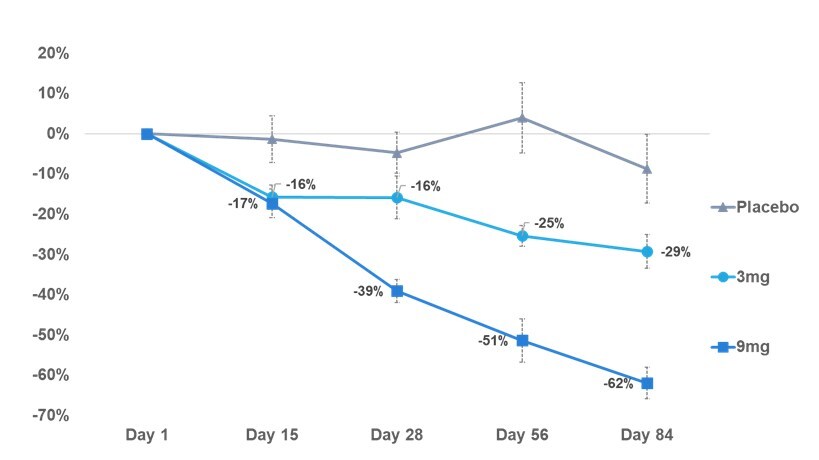
SKY-0515 achieves dose-dependent reductions of mutant huntingtin (mHTT) protein, with 62% lowering at Day 84 on the 9mg daily oral dose
Additional findings include dose-dependent reductions in PMS1 mRNA, excellent brain penetration, and a favorable safety profile
SKY-0515’s Phase 2/3 FALCON-HD trial in patients in Australia and New Zealand is ongoing
BOSTON, Sept. 17, 2025 /PRNewswire/ — Skyhawk Therapeutics, Inc., a clinical-stage biotechnology company developing novel small molecule therapies designed to modulate critical RNA targets, today announced positive results from the first interim analysis of the Part C patient cohort in its Phase 1 clinical trial of SKY-0515, an investigational treatment for Huntington’s disease (HD). At Day 84, patients receiving SKY-0515 demonstrate dose-dependent reductions of mHTT protein in blood including 62% at the 9mg dose. SKY-0515 has been generally well tolerated at both dose levels tested.

Reduction in mHTT protein level in blood from pre-dose through 84 days of treatment. — Skyhawk Therapeutics, Inc. ‘Phase 1 Part C patient cohort reduction in mHTT protein level in blood at Day 84,’ 2025; Note: Error bars represent standard error of the mean. Placebo (N=6), 3mg (N=10), and 9mg (N=10) patient treatment is ongoing. (PRNewsFoto/Skyhawk Therapeutics)
Treatment with SKY-0515 also results in important dose-dependent PMS1 mRNA reduction and demonstrates excellent central nervous system penetration. The overall safety profile has been favorable, supporting the continued clinical development of the program.
“SKY-0515 is reducing mHTT protein to the most impressive extent we’ve seen so far in patients, and crucially the clinical and biomarker data show no safety concerns so far at any dose tested,” said Ed Wild, Professor of Neurology at University College London. “It is great that we are seeing substantial PMS1 reduction as well, which should be a potent combination for treating Huntington’s disease via two of its core pathogenic mechanisms. This is what success looks like at the 3-month timepoint, setting the stage for meaningful impact for people living with HD across the world – for whom an orally administered huntingtin-lowering treatment such as SKY-0515 would be truly transformative.”
“The strength of SKY-0515’s biomarker response after just 84 days of treatment underscores its potential as a transformative therapy for HD,” said Sergey Paushkin, Head of R&D at Skyhawk Therapeutics. “These interim data represent an important milestone for SKY-0515 and highlight the power of Skyhawk’s platform to deliver first-in-class small molecules for devastating diseases with no approved disease-modifying therapies.”
Huntington’s disease is a rare, hereditary, and ultimately fatal neurodegenerative disorder that affects over 40,000 symptomatic patients in the United States, with hundreds of thousands estimated to be affected worldwide. There are currently no approved treatments which slow or halt disease progression. SKY-0515 is Skyhawk’s orally-administered, investigational small molecule RNA splicing modifier developed through the company’s novel RNA-splicing platform, SKYSTAR®. SKY-0515 is designed to reduce both HTT protein and PMS1 protein, an additional key driver of somatic CAG repeat expansion and HD pathology.
SKY-0515 was developed through Skyhawk’s proprietary integrated platform, SKYSTAR®, and is the first Skyhawk drug in clinical trials.
Skyhawk expects to put a series of additional novel drugs to treat rare neurological diseases with no presently approved disease modifying therapies in the clinic by the end of 2027. The first is on track to initiate trials in mid-2026.
About SKY-0515’s Phase 1 Clinical Study
SKY-0515’s Phase 1 clinical trial is a first-in-human trial designed to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics, specifically blood biomarker modulation activity, of SKY-0515 in healthy volunteers and individuals with early-stage Huntington’s disease (HD). The trial is separated into three parts. Parts A and B evaluated SKY-0515 in Healthy Volunteers. Part C is a double-blind placebo-controlled parallel design study of two dose levels of SKY-0515 and placebo of individuals with early-stage HD (HD-ISS Stage 1, 2, or mild Stage 3) for 84 days followed by a 12 month extension of active treatment where all participants will receive either a low or high dose of SKY-0515 in a blinded fashion. The objectives of the study are to evaluate safety, pharmacokinetic, and pharmacodynamic parameters including mutant HTT protein and PMS1 mRNA. Part C enrollment is complete, and topline data from the blinded active treatment extension are expected in mid-2026.
About SKY-0515’s Phase 2/3 FALCON-HD Clinical Study
FALCON-HD (NCT06873334) is a Phase 2/3 randomized, double-blind, placebo-controlled, dose ranging study to evaluate the pharmacodynamics, safety, and efficacy of SKY-0515 in 120 participants with Stage 2 and early Stage 3 HD across 10 sites in Australia and New Zealand. Eligible patients will receive a once-daily oral dose of SKY-0515 at one of three dose levels, or placebo, for a treatment period of at least 12 months. The trial aims to assess the potential of SKY-0515 to modulate RNA splicing and reduce mHTT and PMS1 proteins, which are implicated in the pathology of Huntington’s disease. Additional information about FALCON-HD, including participating sites and eligibility criteria, can be found at ClinicalTrials.gov and www.FALCON-HD.com.
About Skyhawk Therapeutics
Skyhawk Therapeutics is a clinical-stage biotechnology company which uses its proprietary platform, SKYSTAR®, to discover and develop small molecule RNA modulating therapies for the world’s most intractable diseases. For more information visit www.skyhawktx.com.
Skyhawk Contact
Maura McCarthy
[email protected]