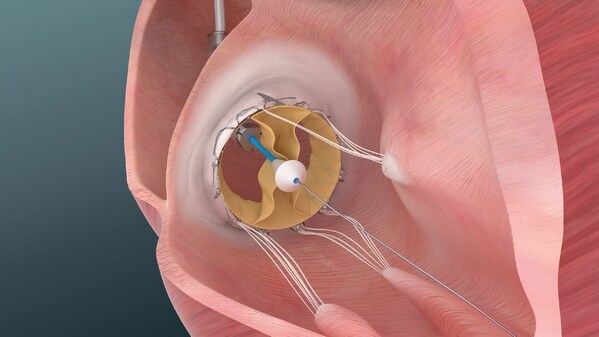
Results demonstrate a favorable safety profile and functional improvements in patients with severe tricuspid regurgitation including patients with reduced right ventricle function
YOKNEAM, Israel, Jan. 21, 2026 /PRNewswire/ — Trisol Medical, a clinical-stage medical device company developing a transcatheter tricuspid valve replacement system, today announced positive results from its FDA-approved U.S. Early Feasibility Study evaluating the Trisol Transcatheter Tricuspid Valve in patients with severe to torrential tricuspid regurgitation.
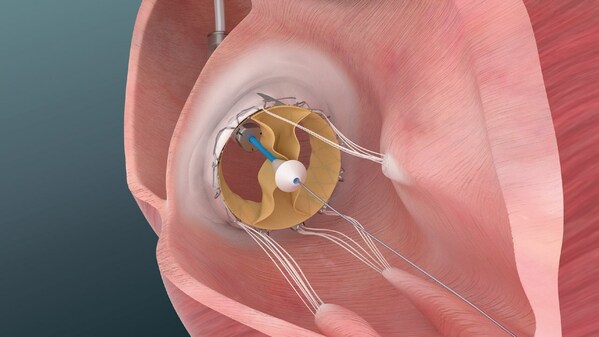
Illustration of Trisol Medical’s transcatheter tricuspid valve replacement system used in the company’s U.S. Early Feasibility Study for patients with severe to torrential tricuspid regurgitation.
Trisol’s Transcatheter Tricuspid Valve Replacement (TTVR) system is designed to treat severe to torrential tricuspid regurgitation (TR), a condition in which the tricuspid valve fails to close properly, allowing blood to flow backward from the right ventricle to the right atrium. Patients with this condition often have limited treatment options.
To date, 22 patients with severe to torrential TR who were considered high risk for conventional surgery have been treated at leading U.S. centers, including Piedmont Heart Institute, Vanderbilt University Medical Center, University of Virginia Health System, Columbia University Medical Center, and Cedars-Sinai Medical Center, using a trans-jugular (TJ) access approach. Enrollment in the TJ cohort is now complete, and the study is proceeding with continued enrollment using Trisol’s newly developed trans-femoral access route.
Key Study Results
- Safety: Less than 5% need for permanent pacemaker at 30-day follow-up.
- Effectiveness: Considerable reduction in TR following implantation.
- Functional outcomes: At 30-day and 12-month follow-up, notable improvements were observed in quality of life (KCCQ), heart failure symptoms (NYHA class), 6-minute walk distance, right ventricular function, and cardiac output.
- Technical performance: Successful deployment of the device and no incidents of migration.
Importantly, these encouraging results include patients with reduced right ventricular function, a large, high-risk subgroup associated with poorer outcomes and underserved by existing and emerging treatment approaches. Early clinical experience reported to date across emerging therapies highlights a persistent unmet need in this patient population, suggesting the Trisol valve could help address this critical gap.
Dr. Pradeep Yadav commented: “Trisol brings several novel features from ease of use to recapturable anchors, broad size range, lower pacemaker rate and performance in dysfunctional right ventricles. We are very excited to investigate the next phase with its transfemoral delivery system and pivotal trial.”
“We are thrilled by these positive outcomes, which further validate the potential of our best-in-class technology to improve care for patients with severe TR. I would like to thank our clinical investigators and the entire clinical teams for their dedication and outstanding patient care,” said Ron Davidson, CEO of Trisol Medical.
Dr. Shimon Eckhouse, Chairman of Trisol, added: “Millions of Americans suffer from TR, and despite recent advances, effective treatment options remain limited for patients with severe TR. The Trisol valve was developed to address this significant unmet need and has the potential to redefine the standard of care.”
About Trisol Medical
Trisol Medical Ltd. is a clinical-stage medical device company specializing in the development of a novel TTVR. Founded within the Alon MedTech Ventures incubator, Trisol is dedicated to advancing minimally invasive heart valve therapies that offer safer alternatives to traditional surgical procedures. For further details, please visit: www.trisol-medical.com.
Media contact:
Ron Davidson
CEO
[email protected]