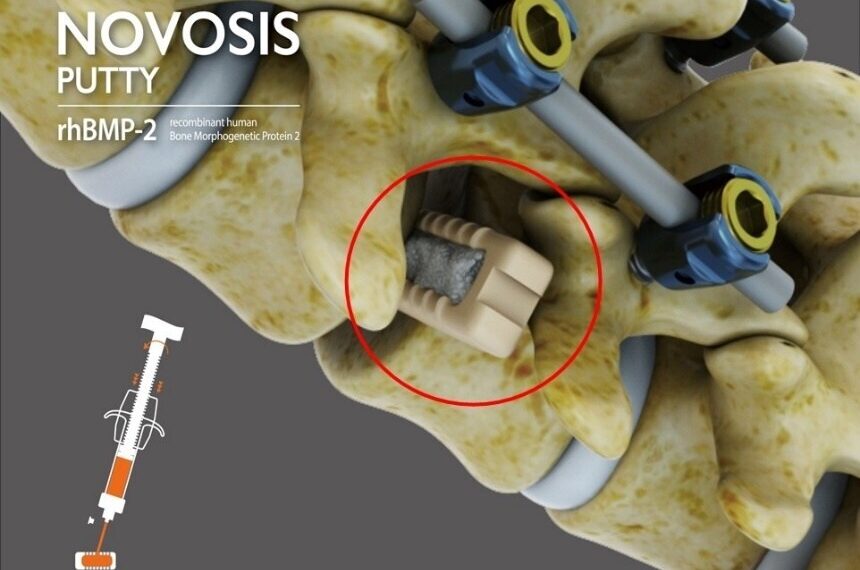
|
– The bone graft incorporating rhBMP-2 has received FDA IDE approval for a pivotal clinical trial in spinal fusion
– Positioned for global expansion in $750M spinal bone graft market
SEOUL, South Korea, April 24, 2025 /PRNewswire/ — CGBIO(CEO Hyun Seung Yu), a leading Korean company specializing in bio-regenerative medicine, proudly announces that its innovative bone graft substitute, NOVOSIS PUTTY, has received Investigational Device Exemption (IDE) approval from the U.S. Food and Drug Administration (FDA). This pivotal approval paves the way for a clinical trial in spinal fusion procedures within the United States.
NOVOSIS PUTTY, previously designated as a Breakthrough Device by the FDA in December 2023, leverages novel technology to address unmet needs in bone regeneration. This milestone marks NOVOSIS PUTTY as the first Korean-developed bio-combined medical device to reach this stage in the U.S., signifying a significant step toward Premarket Approval (PMA) and subsequent commercialization.
The product features a dual-carrier system utilizing Hydroxyapatite (HA) and Tri-Calcium Phosphate (TCP), combined with CGBIO’s proprietary sustained-release technology, SLOREL™, to control the release of recombinant human bone morphogenetic protein-2 (rhBMP-2). This system is engineered to enhance high-density bone formation while minimizing ectopic bone growth, a common adverse effect in earlier rhBMP-2-based products. The safety and efficacy of NOVOSIS PUTTY have been validated in peer-reviewed publications, including the Journal of Clinical Medicine.
The rhBMP-2 protein used in NOVOSIS PUTTY is manufactured by Daewoong Pharmaceutical, a strategic partner of CGBIO. During the IDE application process, the FDA conducted an in-depth review of the manufacturing process and Chemistry, Manufacturing, and Controls (CMC) data, reflecting the FDA’s heightened scrutiny for bio-combined implants.
Jumi Han, Head of U.S. Development at CGBIO USA, overseeing the pivotal clinical program, commented, “Securing IDE approval for this pivotal trial validates our global clinical infrastructure and product quality. We are fully committed to executing a robust clinical trial and expanding NOVOSIS PUTTY’s reach across the U.S. and other major markets”.
Hyun Seung Yu, CEO of CGBIO, stated, “This IDE approval reflects years of consistent R&D efforts and strengthens our global competitiveness. The U.S. bone graft market is notoriously difficult to penetrate, and this achievement underscores the global potential of NOVOSIS PUTTY. We will continue to provide transformative treatment options that improve quality of life for patients worldwide”.
In February 2025, CGBIO and its subsidiary CG MedTech signed a partnership agreement with Johnson & Johnson MedTech for the exclusive supply of NOVOSIS and NOVOSIS TRAUMA products across Korea and other Asian territories. The new IDE approval for NOVOSIS PUTTY is expected to accelerate the global expansion of the entire NOVOSIS product family.
About CGBIO
CGBIO is a global medical device company specializing in advanced biomaterials and regenerative medicine technologies. With a robust product portfolio and numerous successful clinical cases, CGBIO is committed to delivering innovative medical solutions that enhance patient outcomes and quality of life. And to support growing gloval demand, CGBio has begun construction of a new manufacturing facility. While Daewoong Bio has already completed a lare-scale plant dedicated to rhBMP-2 production, ensuring stable supply for the upcomming U.S. and global rollout.